In search of Philosophers Stone
 The Alchemist Discovering Phosphorus depicting Hennig Brand discovering phosphorus
The Alchemist Discovering Phosphorus depicting Hennig Brand discovering phosphorus
Like other alchemists of the time, Hennig Brand (16th Century) searched for the "philosopher's stone", a substance which supposedly transformed base metals (like lead) into gold.
Around 1669 he heated residues from boiled-down Urine on his furnace until the retort was red hot, where all of a sudden glowing fumes filled it and liquid dripped out, bursting into flames. He could catch the liquid in a jar and cover it, where it solidified and continued to give off a pale-green glow. What he collected was phosphorus, which he named from the Greek word for "light-bearing" or "light-bearer."He used about 5,500 litres of urine to produce just 120 grams of phosphorus.
The philosophers' stone
The philosophers' stone was the central symbol of the mystical terminology of alchemy, symbolizing perfection at its finest, enlightenment, and heavenly bliss.
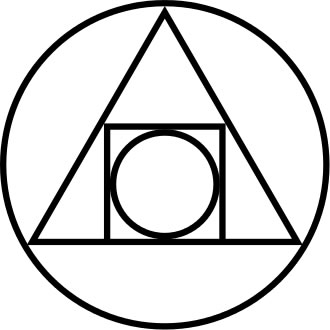 The Squared Circle: an alchemical symbol (17th century) illustrating the interplay of the four elements of matter (earth, fire, water, air) symbolising the philosophers' stone
The Squared Circle: an alchemical symbol (17th century) illustrating the interplay of the four elements of matter (earth, fire, water, air) symbolising the philosophers' stone
Efforts to discover the philosophers' stone were known as the Magnum Opus ("Great Work"). The English philosopher Sir Thomas Browne in his spiritual testament "Religio Medici" (1643) identified the religious aspect of the quest for the philosopher's Stone when declaring: "The smattering I have of the Philosophers stone, (which is something more than the perfect exaltation of gold) hath taught me a great deal of Divinity."
|